Welcome to the Fall Edition of The Glyphosate Renewal Group (GRG) Newsletter
In this edition, we invite you to read about the publication of the full opinion of the European Chemicals Agency (ECHA) on its review of the classification and labelling of glyphosate.
You can also view the updated timeline of the regulatory process following the announcement of the European Food Safety Authority (EFSA) and ECHA on 10 May on the revised timelines for assessments for the renewal of the EU authorisation of glyphosate.
We invite you to learn about a review of EU studies assessing the value of glyphosate to the agriculture sector done by the GRG in collaboration with ADAS.
Finally, we share some facts and figures about the GRG website, now live for over two years, available in nine languages as part of the GRG’s commitment to transparency.
We invite you to connect with us; we listen, and we engage.
Visit our website to learn more, or contact us directly.
___________________________________________________________________________
IN THIS ISSUE
Regulatory Milestones
ECHA published the full opinion of its review of the classification and labelling of glyphosate
Useful Information
Renewal of the EU authorisation of glyphosate: updated timeline of the regulatory process
Socio-economic value of glyphosate- A review of EU studies assessing the value of glyphosate to the agriculture sector
Website News
The GRG website- more than 2 years of unprecedented transparency
___________________________________________________________________________
Regulatory Milestones

ECHA published the full opinion of its review of the classification and labelling of glyphosate
In the first week of July, ECHA published the full Risk Assessment Committee’s (RAC) Opinion on its review of the classification and labelling at EU level of glyphosate.
It follows ECHA’s announcement on May 30th that based on a wide-ranging review of scientific evidence, the RAC concludes that classifying glyphosate as a carcinogen is not justified.
Further, the Committee found that the available scientific evidence did not meet the criteria to classify glyphosate for specific target organ toxicity, or as a mutagenic or reprotoxic substance.
The detailed RAC opinion, which outlines the thorough evaluation can be viewed here.
In addition, ECHA released an 11-page “Explanatory note” on the opinion, available via this link.
As a next step of the regulatory process, the AGG (i.e., the four rapporteur Member States currently assessing glyphosate – Sweden, France, Hungary, and The Netherlands) submitted an updated draft Renewal Assessment Report (dRAR) at the end-September to EFSA in response to the identified scientific topics and following evaluation of the additional information provided by the GRG.
Read the full GRG statement on the Committee’s opinion here.
___________________________________________________________________________
Useful Information
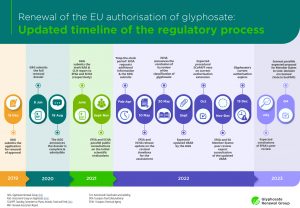
Renewal of the EU authorisation of glyphosate: updated timeline of the regulatory process
On 10 May 2022, EFSA and ECHA published a press release informing that the evaluation for the renewal of the EU authorisation of the active substance glyphosate, as stipulated by the EU Plant Protection Regulation (Regulation (EC) 1107/2009), will be delayed.
Click below for an updated and easy-to-read timeline of the process.
Click here to view the timeline
___________________________________________________________________________

The socio-economic value of glyphosate- A review of EU studies assessing the value of glyphosate to the agriculture sector
The GRG collaborated with ADAS to do a socio-economic meta-analysis of publicly available literature regarding the impact of the loss of glyphosate within the EU.
The aim was to have a clear assessment of the potential implications of a glyphosate ban on EU agriculture, focusing on eight countries that are important producers of agricultural commodities and the EU as a whole, bringing existing information together into a single source.
According to the review, increased tillage practices required to replace glyphosate would negatively impact the environment by increasing soil erosion, threatening biodiversity within soils, and increasing greenhouse gas emissions through higher fuel usage.
An increased fuel consumption per hectare by 15-44 L/ha, resulting in an increase in greenhouse gas emissions of 1.4-3.8 Mt CO2e per year is expected.
Finally, the study underlines that alternative weed control strategies can be logistically challenging to manage as increased labour is required at key points in time, often when the farm is already busy with other activities.
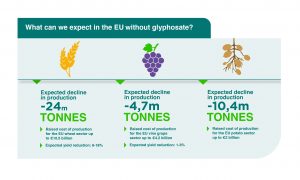
For expected implications on key crops, see the illustration below:
___________________________________________________________________________
Website News

The GRG website- more than 2 years of unprecedented transparency
To respond to the demand for greater transparency and information on the process and all its constitutive elements, in spring 2020, the GRG launched the GRG website.
In August 2020, the firstly translated versions, German, and French, went live. Today, the website is available in 9 languages, reflecting the GRG’s commitment to maximum transparency.
Since the launch, approximately 130k users from all over Europe visited the website, with most visitors from France, Poland, Germany, Spain, and Italy.
The pages drawing the most attention were the Useful Information, FAQs, and the (EU) Authorisation Process pages. The most searched terms were ‘glyphosate’, ‘the AGG’ (Assessment Group on Glyphosate), and ‘biodiversity’.
We constantly share updates and publish additional information on the website as we stay attuned to the questions and topics of interest.
We invite you to visit our website, a one-stop shop for all information about glyphosate and the ongoing process of renewal of the EU authorization of the active substance, to get answers to all your questions or contact us directly via the get-in-touch form.
___________________________________________________________________________
Would you like to receive the newsletter via email? Subscribe here.