The Glyphosate Dossier Is Now Available: We Listened, We Learnt
We are proud to announce that the scientific dossier, submitted as part of the EU glyphosate reapproval process, is now available publicly. With this unprecedented step, bringing greater transparency to the EU regulatory process, the Glyphosate Renewal Group further delivers on its Transparency Commitments. Find more about our commitment to transparency here.
Via this website, the Glyphosate Renewal Group grants unrestricted access to the various parts of the robust scientific dossier submitted on 8 June 2020 to the Assessment Group on Glyphosate (AGG)* and proving that glyphosate does not pose a risk to human health and the environment when used in accordance with the label instructions.
In addition to this, the Glyphosate Renewal Group has already made public the scientific dossier submitted in 2012 for the previous authorisation and all the official documents of the 2020 regulatory process: the application, the minutes of the meetings and the correspondence with the Authorities from AGG.
The AGG will now carry out a science-based evaluation of the complete dossier with the aim of issuing a Renewal Assessment Report (RAR), with its conclusions on glyphosate safety when used in accordance with the label instructions for human health and the environment, scheduled to be published before July 2021.
What does the 2020 scientific dossier include?
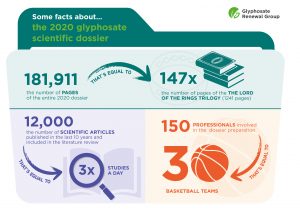
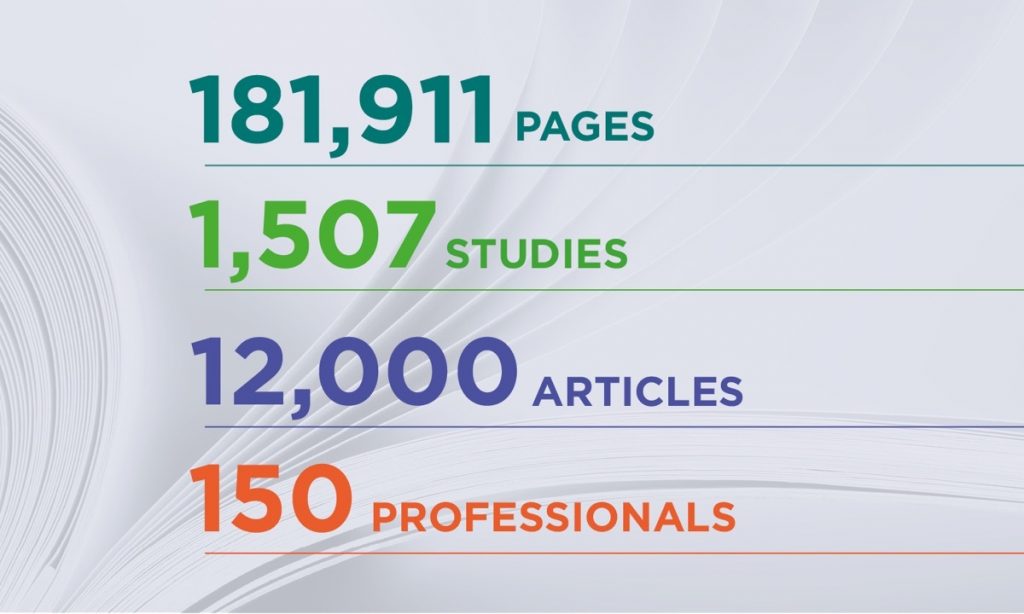
The dossier submitted on 8 June to the AGG includes scientific evidence on glyphosate safety:
- Study reports: there are approximately 1,500 scientific studies in the 2020 dossier, out of which 100 are new. It is possible to obtain a copy of the reports of those studies by filling in the online order form (click here). If you wish to receive all the study reports, please contact us. For data privacy and in accordance with existing law, all personal information and testing facilities are redacted from the documents.
- Summaries of the studies: these documents include information such as the test setup, test execution and results, Good Laboratory Practice (GLP), equations and explanations from guidelines, software outputs, method validation reports, certificates and data tables. Find the summaries here.
- Public literature: this includes an evaluation of around 12,000 published scientific peer-reviewed articles on glyphosate and its relevant metabolites, published within the last 10 years. Since those studies were not conducted by the Glyphosate Renewal Group or its members, we can only list them, but we are not allowed to reproduce them for copyright reasons. Find the public literature here.
*The AGG is composed of the regulatory authorities of France, Hungary, The Netherlands and Sweden, acting jointly as Rapporteurs for the glyphosate dossier.